Answer:
This is because of the solubility of the reacting metal salts.
Step-by-step explanation:
The use of solubility of salts influences the formation of precipitates. Most precipitation reactions occur as a result of the replacement reactions or double placement reactions. A double replacement reaction occurs when two ionic reactants dissociate and bond with respective anion and cation of the other reactant. This is simply a switch over reaction.
A double replacement reaction occurs when a chemical reaction occurs and one of the products formed is insoluble.
Here is an example:
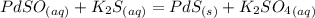
Both reactants are acqeous and the product is solid.