Answer:- 6
Solution:- The given hypothetical equation is:
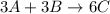
From this equation, there is 3:6 mol ratio between A and C.
From given info, 3 moles of A reacts with excess of B to produced C. It asks, how many moles of C are produced.
Since the mol ratio between A and C is 3:6 means 3 moles of A produce 6 moles of C.
We multiply the given number of moles of A by the mol ratio.
So,
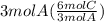
= 6 mol C
It wants answer without the unit. So, the answer is 6.