Consider the reaction:
2 H₂O₂ =>2 H₂O + O₂
2 moles of H₂O₂ will give=2 moles of H₂O and 1 mole of O₂
1 moles of H₂O₂ will give=1 moles of H₂O and 0.5 mole of O₂
31.5 g of H₂O₂=
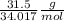
=
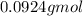
0.0924 moles of H₂O₂ will give= 0.5 × 0.0924 mole of O₂
=0.462 mole of O₂
0.462 mole of O₂×32 g/mol
14.784 g of O₂
If 31.5 grams of hydrogen peroxide reacts to form 16.7 grams of water, 14.784 g of oxygen must simultaneously be formed