Answer:
0.967mole
Step-by-step explanation:
Given parameters:
Volume of NH₄Cl = 21.67L
Unknown:
Number of moles = ?
Solution:
If we assume that the volume was taken at standard temperature and pressure,
Then;
Number of moles =
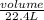
Number of moles =
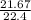 = 0.967mole
= 0.967mole