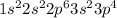Answer : The electronic configuration of sulfur will be,
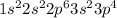
Explanation :
Electronic configuration : It is defined as the arrangement of electrons of a molecule in a molecular orbital.
Element = Sulfur
Atomic number = 16
The number of electrons is equal to the atomic number.
So, the number of electrons = 16
The electronic configuration of sulfur will be,