Answer:- B. 4.65 g.
Solution:- The given balanced equation is:
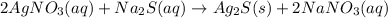
It asks to calculate the mass of silver sulfide formed by when 0.0150 liters of 2.50 M of silver nitrate are used.
Moles of silver nitrate are calculated on multiplying it's liters by its molarity and then on multiplying by mol ratio, the moles of silver sulfide are calculated. These moles are multiplied by the molar mass to convert to the grams.
Molar mass of
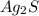 = 2(107.87)+32.06 = 247.8 g per mol
= 2(107.87)+32.06 = 247.8 g per mol
The dimensional set up for the complete problem is:
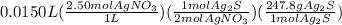
=
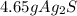
So, the correct choice is B. 4.65 g.