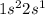Answer : The electronic configuration of atomic number 3 will be,
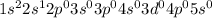
Explanation :
As we are given that the atomic number of an element is, 3. Now we have to determine the electronic configuration of an element that has the atomic number 3.
As we know that the atomic number is equal to the number of protons or number of electrons.
So, the number of electrons is equal to 3.
Electronic configuration : It is defined as the arrangement of electrons of an atoms or ions in an atomic orbital.
Therefore, the electronic configuration of atomic number 3 will be,