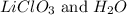Answer: The products are
Explanation: The reaction of acid and base is considered as a neutralization reaction. In this chemical reaction, acid reacts with base to form a salt and water.

For the reaction of
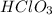 and LiOH, the equation follows:
and LiOH, the equation follows:
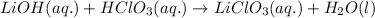
By stoichiometry,
1 mole of Lithium hydroxide reacts with 1 mole of Chloric acid to produce 1 mole of Lithium chlorate and 1 mole of water.
Hence, the products are