Answer: C. The acidity of acid rain is greater than the acidity of natural rainwater.
Step-by-step explanation: Acid rain is caused by the oxides of sulfur and nitrogen which when react with water lead to formation of acids and when combines with rain is called as acid rain.

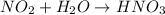
pH is the measure of acidity or alkalinity. More is the amount of
 ions, lesser is the pH and more is the acidity.
ions, lesser is the pH and more is the acidity.