Here we have to get the equation or process by which the unknown concentration of HCl can be determined by the known concentration of NaOH.
The equation to determine the unknown concentration will be
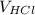 ×
×
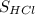 =
=
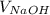 ×
×
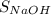 .
.
The volume of the HCl or titrant will be known as it will be taken in the conical flask and the volume of the titre i.e. NaOH will be taken in burret. The indicator used in this titration is phenlopthalien.
At the end point the color of the colorless solution will become light pink and from the burret reading we can get the volume of NaOH required in the titration process.
On plugging the volume, strength of NaOH and volume of HCl, we can obtain the strength of HCl.