Answer:
Option A.
Step-by-step explanation:
To decrease pressure by increasing volume, the equilibrium of the reaction shift to the left as the reactant side has greater number of moles than the product side.
Equilibrium also shifts to the left if temperature decreases.
Given equation is
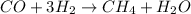
In this case, equilibrium shifts to the left on adding heat to the product mixture .