Answer :
Solution For 1 :
Given,
wavelength =
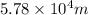
Speed of light =
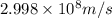
Formula used :
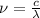 .............(1)
.............(1)
 = frequency
= frequency
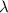 = wavelength
= wavelength
c = speed of light
Now put all the given values in above formula, we get
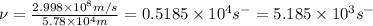 =
=
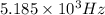
(1Hz = 1 per second)
Therefore, the frequency of a photon in hertz is
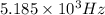 .
.
Solution For 2 :
Given,
Frequency =
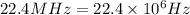
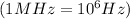
Speed of light =
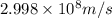
Now put all the given values in above formula (1), we get
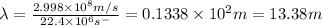
Therefore, the wavelength of a wave is 13.38 m.
Solution For 3 :
Given,
wavelength =
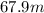
Speed of light =
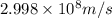
Now put all the given values in above formula (1), we get
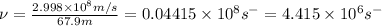 =
=
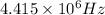
Therefore, the frequency of a photon in hertz is
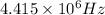 .
.
Solution For 4 :
Given,
Frequency =
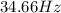
Speed of light =
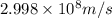
Now put all the given values in above formula (1), we get
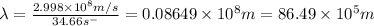
Therefore, the wavelength of a wave is
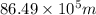 .
.
Solution For 5 :
Given,
wavelength =
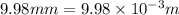 (1 m = 1000 mm)
(1 m = 1000 mm)
Speed of light =
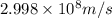
Now put all the given values in above formula (1), we get
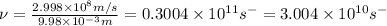 =
=
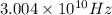
Therefore, the frequency of a photon in hertz is
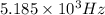 .
.