The correct answer is: Option (A) 448.955 kPa
Step-by-step explanation:
The formula for the absolute pressure is given as:
Absolute-pressure = Gauge-pressure + Atmospheric-pressure

Now rearrange the above equation to find the gauge pressure:
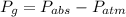
Insert the values:
Absolute pressure =
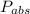 = 550.280 kPa
= 550.280 kPa
Atmospheric pressure =
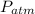 = 101.325 kPa
= 101.325 kPa
Gauge pressure =
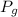 = ?
= ?
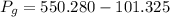
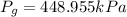
Hence the correct answer is: Option (A) 448.955 kPa