Answer:
14) Reactant
15) 1 .Precipitation reaction equation
16) The coefficient
17) 6.6242 × 10²⁴ atoms of oxygen
18) The product
20) The four states of matter are;
a) Solids
b) Liquids
3) Gas
4) Plasma
21) The total number of atoms in the product is 2.7099 × 10²⁵ atoms.
Step-by-step explanation:
14) The left side of the chemical equation is the reactant
15) The coefficient is the number in font of the formula, here, the coefficient of the reactant C₁₂H₂₂O₁₁ is 1 .This is a precipitation reaction equation
16) The number 11 in front of the formula, H₂O, is called a coefficient
17) On mole of a substance is defined as containing one Avogadro's number,
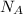 of molecules
of molecules
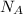 ≈ 6.022 × 10²³ mol⁻¹
≈ 6.022 × 10²³ mol⁻¹
In the reactant, C₁₂H₂₂O₁₁, there are 11 elements of oxygen in one mole of the reactants, which gives 11 × 6.022 × 10²³ = 6.6242 × 10²⁴ atoms of oxygen in one mole of the reactants
18) The right side of the chemical equation is called the product
20) The four states of matter are;
a) Solids
b) Liquids
3) Gas
4) Plasma
21) The number of elements on the product side are;
12 Carbon + 22 Hydrogen + 11 Oxygen = 45 Elements total
However, given that there are 12 moles of carbon, we have;
12 × 6.02 × 10²³ = 7.2264 × 10²⁴ atoms of carbon
For hydrogen, we have;
22 × 6.02 × 10²³ = 1.32484 × 10²⁵ atoms of hydrogen
For oxygen, we have;
11 × 6.02 × 10²³ = 6.6242 × 10²⁴ atoms of oxygen
The total number of atoms in the product is 7.2264 × 10²⁴ + 1.32484 × 10²⁵ + 6.6242 × 10²⁴ = 2.7099 × 10²⁵ atoms.