Answer: Concentration of NaOH calculated will be underestimated.
Step-by-step explanation:
End point is an observational point , which tells us about the completion of reaction between the titrant (solution in burette) and titre(solution in conical flask) in titration experiment.
In this case , NaOH is titrant whose concentration is unknown.
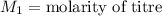 ,
,
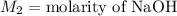
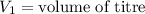 ,
,
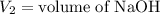
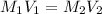
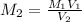 ....(1)
....(1)
According to question a chemist overshoots the end point and adds to much of NaOH solution, which means increase in the value of
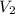 .
.
Then the value of
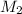 in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH.
in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH.