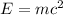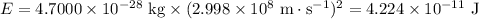Answer:
4.993 ×10⁻¹¹ J
Step-by-step explanation:
The nuclear binding energy is the energy equivalent to the mass defect.
The mass defect is the difference between the mass of a nucleus and the sum of the masses of its nucleons.
Calculate the mass defect
16 p = 16 × 1.007 28 u = 16.116 48 u
16 n = 16 × 1.008 67 u = 16.138 72 u
Total mass of nucleons = 32.255 20 u
- Mass of S-32 = 31.972 070 u
Mass defect = 0.283 13 u
Convert the unified atomic mass units to kilograms.
Mass defect
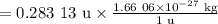
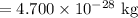
Use Einstein’s equation to convert the mass defect into energy