Step-by-step explanation:
A molecule is defined as the substance that contains same or different elements which are chemically combined to each other in a fixed ratio by mass.
For example,
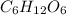 is a molecule.
is a molecule.
And, the stoichiometric coefficient placed before a molecule represents how many total molecules of that substance are actually present.
For example, in
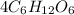 , the stoichimetric coefficient is 4 and it depicts that there are 4 molecules of
, the stoichimetric coefficient is 4 and it depicts that there are 4 molecules of
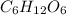 are present.
are present.
Therefore, total number of each item present in
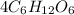 are as follows.
are as follows.
- Carbon atoms :
 = 24
= 24
- Hydrogen atoms :
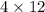 = 48
= 48
- Oxygen atoms :
 = 24
= 24
Thus, we can conclude that in 4 molecules of
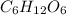 there are 24 carbon atoms, 48 hydrogen atoms and 24 oxygen atoms.
there are 24 carbon atoms, 48 hydrogen atoms and 24 oxygen atoms.