Answer: Law of definite proportion.
Step-by-step explanation: Law of definite proportion states that any chemical compound consists of elements in a fixed ratio by their masses. This law is also known as Proust's Law.
We are given a chemical compound

Assuming that the total mass of 1 mole of
 = 100 grams
= 100 grams
where
Mass of Sodium = 27.37 grams
Mass of Hydrogen = 1.20 grams
Mass of Carbon = 14.30 grams
Mass of Oxygen = 57.14 grams
To Calculate the ratio of all the elements, we divide each of the masses by the lowest mass.
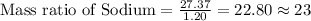
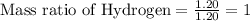
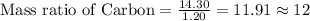
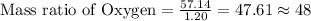
Mass ratio of elements in
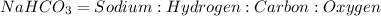
Na : H : C : O = 23 : 1 : 12 : 48
For every
 compound, this mass ratio will always be constant.
compound, this mass ratio will always be constant.