Answer: The concentration of chlorate ion is 0.467 M
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
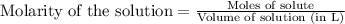 .....(1)
.....(1)
- For
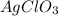 :
:
Molarity of
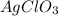 solution = 0.393 M
solution = 0.393 M
Volume of solution = 925 mL = 0.925 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
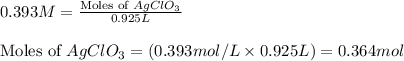
- For
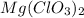 :
:
Molarity of
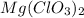 solution = 0.283 M
solution = 0.283 M
Volume of solution = 685 mL = 0.685 L
Putting values in equation 1, we get:
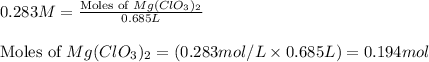
The chemical equation for ionization of silver chlorate follows:
1 mole of silver chlorate produces 1 mole of silver ion and 1 mole of chlorate ion
Moles of chlorate ion = 0.364 moles
The chemical equation for ionization of magnesium chlorate follows:
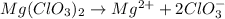
1 mole of magnesium chlorate produces 1 mole of magnesium ion and 2 moles of chlorate ion
Moles of chlorate ion = (2 × 0.194) = 0.388 moles
- Now, calculating the molarity of chlorate ion by using equation 1, we get:
Moles of chlorate ion = (0.364 + 0.388) = 0.752 moles
Volume of solution = (925 + 685) = 1610 mL = 1.610 L
Putting values in equation 1, we get:
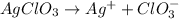 0.752
0.752

Hence, the concentration of chlorate ion is 0.467 M