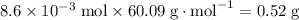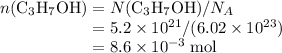
where
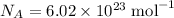 the Avogadro's constant that relates the number of particles to their number, in the unit moles
the Avogadro's constant that relates the number of particles to their number, in the unit moles
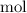 .
.
The molar mass of propanol- mass per mole propanol- can be directly deduced from its molecular formula with reference to a modern periodic table.
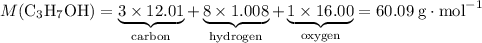
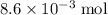 of propanol molecules would thus have a mass of
of propanol molecules would thus have a mass of