S orbital.
Group 1 elements have a general configuration
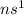 , where n represents the highest occupied Principal Energy Level. For example, Lithium has the valence configuration
, where n represents the highest occupied Principal Energy Level. For example, Lithium has the valence configuration
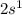 whereas Cesium has
whereas Cesium has
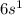 . Both of them belong to Group 1 of Periodic Table.
. Both of them belong to Group 1 of Periodic Table.
Group 2 elements have a general configuration of
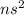 . For example, Magnesium has
. For example, Magnesium has
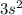 as its outer shell configuration while Strontium has the same as
as its outer shell configuration while Strontium has the same as
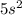 .
.
We see that in both the cases, the outermost S orbital is being filled.