Answer
A second container has 1000 ml of water will contan more thermal energy
Step-by-step explanation
As thermal energy can be determine as
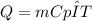
Cp and ΔT will be same for both of the container as both have water and same temperature.
At the condition of same temeprtaure and object, the object with greater volume or mass has greater thermal energy
So,
1st container has 100ml, while 1000ml in 2nd container.
The Thermal energy of 2nd container, having 1000ml water will be greater.