Answer: The percentage of R-enantiomer is 62 %, the percentage of S-enantiomer is 38 % and the percent enantiomer excess is 24 %
Step-by-step explanation:
We are given:
A chiral molecule limonene which is 62 % enantiopure.
This molecule has two enantiomers, which are R-limonene and S-limonene.
Let us consider that 62% of the given molecule is R-limonene.
So, the remaining S-limonene will be = (100 - 62) = 38 %
Percent enantiomeric excess is defined as the difference between the percentage major enantiomer and the percentage minor enantiomer.
Mathematically,
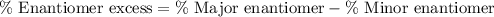
% major enantiomer = 62 %
% minor enantiomer = 38 %
Putting values in above equation, we get:
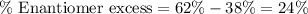
Hence, the percentage of R-enantiomer is 62 %, the percentage of S-enantiomer is 38 % and the percent enantiomer excess is 24 %