Step-by-step explanation:
A stoichiometric coefficient present in front of an atom in both reactant and product side represents the molecules of that atom participating in the reaction.
Whereas number written on the lower left end of a molecules displays the number of atoms.
For example, in
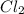 molecule there are 2 chlorine atoms attached to each other.
molecule there are 2 chlorine atoms attached to each other.
On the other hand, moles also represent the stoichiometric coefficient in front of a molecule.
For example,
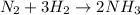
Therefore, we can conclude the following.
- There are 3 molecules of H₂ are in the reactants.
- There are 2 nitrogen atoms are present on reactant side.
- There is 2 moles of NH₃ formed.