Part 1:
The accuracy of a measurement is defined as the closeness of experimental results to the true value. The true value given to us is 27.20 g . From the experimental results we can see that the mass of the cylinder is in the range of 25 which is not very close to the actual value. This indicates that the measurements are not very accurate
Whereas, precision of a measurement is the closeness of experimental values to each other. The reading are close to each other. Therefore we can say that the measurements are precise.
Part 2:
The formula to calculate % error is given as
Percent error =
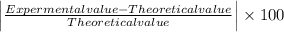
Percent error =
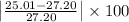
Percent error = 8.05%
Percent error for the Milhouse's first weighing was 8.05%