Answer: Number of Moles of base required in the pill will be 0.5 moles.
Step-by-step explanation: An antacid is a solution of base generally made of
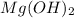 . An antacid pill is used in the case of acidity to neutralize the excessive acid content formed in stomach.
. An antacid pill is used in the case of acidity to neutralize the excessive acid content formed in stomach.
Number of moles of acid = 0.5 moles.
Number of moles of base required to neutralize the acid = Number of moles of acid.
Number of moles of base = 0.5 moles.