Answer:
5.14mole
Step-by-step explanation:
Given parameters:
Mass of sodium bicarbonate = 431.56g
Unknown:
Number of moles = ?
Solution:
To find the number of moles, we use the expression below:
Number of moles =
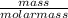
Molar mass = 84.01g/mol
Number of moles =
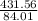 = 5.14mole
= 5.14mole