No. The reaction is not a redox reaction.
Redox reaction is defined as a chemical reaction in which both oxidation and reduction of chemical species takes place. In such reactions, one of the chemical species undergoes reduction and other undergoes oxidation.
Here, oxidation means increase in oxidation state and reduction means decrease in the oxidation state.
The given reaction is as follows:
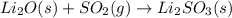
Here, the most stable oxidation state of oxygen is -2. Calculate the oxidation state of Li and S before and after the reaction completion.
On reactant side:
Let the oxidation state of Li in
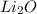 be x thus,
be x thus,
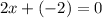
Or,
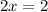
Or,
x=+1
Similarly let the oxidation state of S in
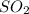 be y thus,
be y thus,
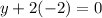
Or,
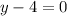
Or,
y=+4
Thus, oxidation state of Li and S on reactant side is +1 and +4 respectively.
Now, the product formed is
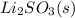 , it is an ionic compound thus it contains
, it is an ionic compound thus it contains
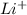 and
and
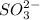 ions.
ions.
Thus, the oxidation state of Li will be +1.
Calculate the oxidation state of S in
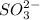 .
.
Let the oxidation state be z thus,
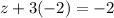
Or,
z=-2+6=+4
The oxidation state of Li and S on the product side is also +1 and +4 respectively.
Since, the oxidation state of any of the chemical species is not changed (no oxidation or reduction take place), the reaction is not a redox reaction.