Answer:
220g
Step-by-step explanation:
The reaction equation is given as:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Number of moles of ethane gas = 2.5moles
To find the mass of CO₂ produced, we need to know the number of moles of the compound produced;
2 mole of ethane gas produced 4 moles of CO₂
2.5 moles of ethane gas will produce
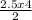 = 5mole of CO₂
= 5mole of CO₂
So;
Mass of CO₂ = number of moles x molar mass
Molar mass of CO₂ = 12 + 2(16) = 44g/mol
Mass of CO₂ = 5 x 44 = 220g