Answer : The weight of compound y would be removed is 3.6363 g.
Solution : Given,
Distribution coefficient, K = 10
Volume of ether (organic layer) = 100 ml
Volume of water (aqueous layer) = 100 ml
Total Weight of material dissolved = 4 g
Formula used :
![K=([Solute]_(o) )/([Solute]_(aq))=(C_(o) )/(C_(aq))=(W_(o)/V_(o) )/(W_(aq)/V_(aq) )](https://img.qammunity.org/2019/formulas/chemistry/high-school/koa92t0hscdkg41b9ntkd36fgfiou0pvzp.png)
Where,
'O' repersent for organic layer and 'aq' repersent for aqueous layer.
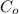 and
and
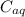 are the concentration of organic solution and the concentration of aqueous solution respectively.
are the concentration of organic solution and the concentration of aqueous solution respectively.
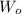 and
and
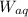 are the weight of material dissolved in each organic and aqueous layer respectively.
are the weight of material dissolved in each organic and aqueous layer respectively.
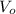 and
and
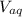 are the volume of organic and aqueous layer respectively.
are the volume of organic and aqueous layer respectively.
Let, the weight of compound y removed '
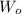 ' is 'X' gram.
' is 'X' gram.
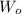 = X gram
= X gram
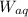 = Total weight of material - weight of compound y removed = 4 - X gram
= Total weight of material - weight of compound y removed = 4 - X gram
Now put all the values in above formula,we get
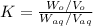
10=
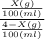
Rearranging the terms, we get the value of X, as
X = 3.6363 g