Answer:
The thickness in millimeters of the is 0.02552.
Step-by-step explanation:
Area of an aluminum foil = A =
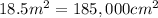
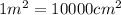
Mass of the assuming foil = 1275 g
Volume of the aluminum foil = V
Thickness of the assuming foil = h
Density of the aluminum foil =
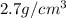
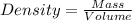
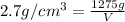
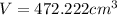
Volume = Area × Depth
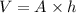
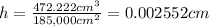
1 cm = 10 mm
h = 0.02552 mm
The thickness in millimeters of the is 0.02552.