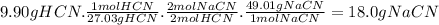Answer:
18.0 g
Step-by-step explanation:
There is some info missing. I think this is the original question.
Hydrogen cyanide, HCN, is a poisonous gas. The lethal dose is approximately 300 mg HCN per kilogram of air when inhaled. The density of air at 26 °C is 1.18 kg/m³.
Consider the formation of HCN by the reaction of NaCN (sodium cyanide) with an acid such as H₂SO₄ (sulfuric acid):
2 NaCN(s) + H₂SO₄(aq) → Na₂SO₄(aq) + 2 HCN(g)
What mass of NaCN gives the lethal dose in the 28.0 m³ room? Express your answer to three significant figures and include the appropriate units.
The density of air is 1.18 kg/m³. The mass represented by 28 m³ is:
28.0 m³ × (1.18 kg/m³) = 33.0 kg
The lethal dose is approximately 300 mg HCN per kilogram of air. The lethal dose of HCN in 33.0 kg of air is:
33.0 kg air × (300 mg HCN / 1 kg air) = 9900 mg HCN = 9.90 g HCN
From the balanced equation, we can establish the following relations:
- The molar mass of HCN is 27.03 g/mol.
- The molar ratio of HCN to NaCN is 2:2.
- The molar mass of NaCN is 49.01 g/mol.
The mass of NaCN that produces 9.90 g of HCN is: