In 1 mole of
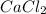 , there are 3 moles of ions, 1 mole of Ca^{2+} and 2 mole of
, there are 3 moles of ions, 1 mole of Ca^{2+} and 2 mole of
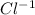 .
.
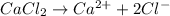
Molar mass of
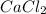 is 110.98 g/mol. Calculating number of moles from given mass as follows:
is 110.98 g/mol. Calculating number of moles from given mass as follows:
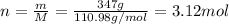
Thus, number of moles of ions will be
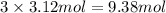 .
.
Since, 1 mole of any substance has
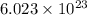 units of that substance where
units of that substance where
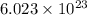 is Avogadro's number.
is Avogadro's number.
Thus, 9.38 mol of ions will have
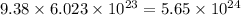 number of ions.
number of ions.
Therefore, total number of ions in 347 g of
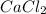 is
is
 .
.