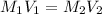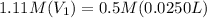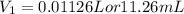Concentration of the given stock solution of glucose = 20% (m/v)
It means that 20 g glucose is present in 100 mL solution
Volume of the 20 % solution given is 84 mL
Moles of glucose =
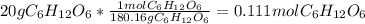
Moles of glucose in 84 mL solution:
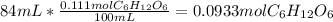
Calculating molarity of 84 mL of 20 % glucose solution:
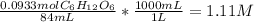
Finding the volume of this solution required to prepare 0.0250L of 0.5 M glucose solution: