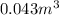Answer:
Step-by-step explanation:
As we know that volume is equal to the mass divided by density.
Mathematically, it can be represented as
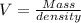
Where V represents the volume of substance
Substituting the value of mass and density in the above equation, we get
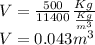
Thus, the volume of lead is