Answer : The Molar mass of the compound Di-nitrogen peroxide i.e
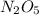 = 108.02 g/mol
= 108.02 g/mol
Solution :
Molar mass of
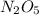 ,
,
Step 1 : Identify the number of atoms of each element in the compound.
Number of Nitrogen atoms (N) = 2
Number of Oxygen atoms (O) = 5
Step 2 : Find the atomic mass of each element in the compound.
Atomic mass of Nitrogen (N) = 14.01 g/mol
Atomic mass of Oxygen (O) = 16.00 g/mol
Step 3 : Calculate the Molar mass of the given compound
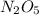 .
.
Molar mass of
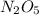 =
=
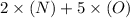
=
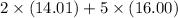
= 108.02 g/mol