Answer:- 9.4 minutes.
Solution:- Radioactive decay obeys first order reaction kinetics and the equation used to solve this type of problems is:
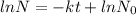
where, k is decay constant and t is the time.
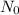 is the initial amount of the radioactive substance and N is the remaining amount.
is the initial amount of the radioactive substance and N is the remaining amount.
Since the value of decay constant is not given, so we need to calculate it first from given half life by using the formula:
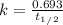
where
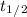 stands for half life.
stands for half life.
Given half life is 3.0 minutes.
So,
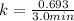
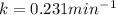
Let's plug in the values in the first order reaction equation and solve it for t.
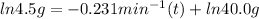
It could also be written as:
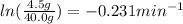
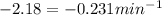
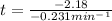
k = 9.4 min
So, the radioactive substance would take 9.4 minutes to decay from 40.0 grams to 4.5 grams.