Answer : The number of moles of nitrogen are 0.00840 moles.
Explanation : Given,
Mass of
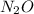 = 0.185 g
= 0.185 g
Moles of
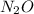 = 44 g/mole
= 44 g/mole
First we have to calculate the moles of
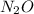 .
.
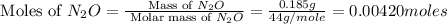
Now we have to calculate the moles of nitrogen (N).
In
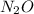 , there are 2 moles of nitrogen atom and 1 mole of oxygen atom.
, there are 2 moles of nitrogen atom and 1 mole of oxygen atom.
As, 1 mole of
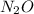 contains 2 moles of nitrogen
contains 2 moles of nitrogen
So, 0.00420 mole of
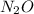 contains 2 × 0.00420 = 0.00840 moles of nitrogen
contains 2 × 0.00420 = 0.00840 moles of nitrogen
Therefore, the number of moles of nitrogen are 0.00840 moles.