Complete combustion of naproxen takes place in excess of oxygen thus, naproxen is a limiting reactant and amount of carbon dioxide produced from it is theoretical yield of carbon dioxide.
The balanced chemical reaction for combustion of naproxen is as follows:
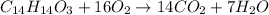
From the balanced chemical reaction, 1 mole of naproxen
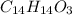 gives 14 mol of
gives 14 mol of
 .
.
The mass of naproxen is given 250 g, molar mass is 230.259 g/mol thus, number of moles will be:
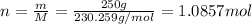
Thus, number of moles of
 obtained from 1.0857 mol of naproxen will be:
obtained from 1.0857 mol of naproxen will be:
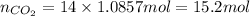
Now, convert this into mass as follows:
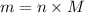
Molar mass of
 is 44 .01 g/mol thus,
is 44 .01 g/mol thus,
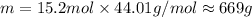
Thus, theoretical yield of
 is 669 g.
is 669 g.