Given the volume % of isopropyl alcohol = 36.0 % (v/v)
36.0 mL of isopropyl alcohol is present in 100 mL solution
Volume of the solution = 675 mL
Calculating the volume of isopropyl alcohol present in 675 mL solution:
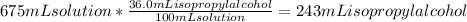
Volume of isopropyl alcohol in 675 mL solution = 243 mL
Volume of solute (isopropyl alcohol)+Volume of solvent(water) = Volume of the solution
Volume of water present in the solution = 675 mL - 243 mL =432 mL