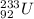The given isotope of Neptunium is
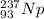
Alpha decay of an isotope results in daughter nuclide with mass number less by 4 units and atomic number less by 2 units than the parent isotope.
Alpha decay of Neptunium-237 can be represented as:
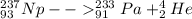
Beta decay of the above formed protactinium nuclide can be represented as:
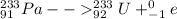
Gamma decay releases only energy in the form of gamma rays, the nuclide remains the same.
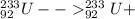 γ
γ
The new atom formed after the given decays is