Answer:- C. 16.4 L
Solution:- The given balanced equation is:
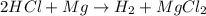
From this equation, there is 2:1 mol ratio between HCl and hydrogen gas. First of all we calculate the moles of hydrogen gas from given grams of HCl using stoichiometry and then the volume of hydrogen gas could be calculated using ideal gas law equation, PV = nRT.
Molar mass of HCl = 1.008 + 35.45 = 36.458 gram per mol
The calculations are shown below:
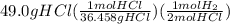
=
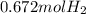
Now we will use ideal gas equation to calculate the volume.
n = 0.672 mol
T = 25 + 273 = 298 K
P = 101.3 kPa = 1 atm
R =
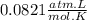
PV = nRT
1(V) = (0.672)(0.0821)(298)
V = 16.4 L
From calculations, 16.4 L of hydrogen gas are formed and so the correct choice is C.