The metal is being oxidized.
Metals- along with other elements- are neutral and have oxidation state of
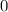 in their respective elementary states.
in their respective elementary states.
Metal atoms can lose electrons to electronegative elements such as oxygen on exposure. Atoms that have lost one or more negatively-charged electrons are no longer neutral and demonstrate a positive charge. Those atoms have converted to positively charged ions or cations.
Oxidation is the process of losing electrons whereas Reduction refers to the process of gaining electrons. "OIL RIG." Metal atoms here lose electrons and are thus oxidized.