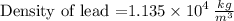
Dimension of rectangular ingot = 2.00 cm × 2.00 cm × 0.850 dm
1dm = 10cm
=> 0.850dm = 0.850×10 = 8.50 cm
Volume of ingot = 2.00 cm × 2.00 cm × 8.50 = 34.00 cm^3
1 m^3 = (100)^3 cm^3 = 10^6 cm^3
1 cm^3 = 1/10^6 = 10^(-6) m^3
34.00 cm^3 = 34 × 10^(-6) = 3.4 × 10^(-5) m^3
_____________-
Volume = 3.4×10^(-5) m^3
density = 1.135 × 10^4 kg/m^3
mass = density × volume =1.135 × 10^4 × 3.4×10^(-5) = 0.3859 kg
__________________
1kg = 1000g
0.3859 kg = 0.3859×1000 = 385.9 gram.
mass is 385.9 gram.