Mass and energy are inter convertible.As per Einsteins mass and energy relations i.e
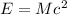 ,some amount of energy is lost that is converted into energy .
,some amount of energy is lost that is converted into energy .
In our day to day life ,generally mass is lost and energy is formed. In a ideal assumption we subtract the mass of earlier one from the later one which indirectly denotes that some amount of mass is lost and energy is released .There is also endothermic reactions where energy is needed which does not mean mass is added.Here simply energy is needed to initiate the reactions.
So instead of subtracting the smaller value from lager we subtract the initial value from final as the latter one can explain a physical phenomenon in a better way.we can get some in formation from it.