The formula of number of moles is:
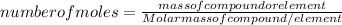 -(1)
-(1)
Mass of
 = 440 mg (given)
= 440 mg (given)
Since, 1 mg = 0.001 g
So, 440 mg = 0.44 g
Molar mass of
 =
=
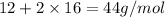
Substituting the values in formula (1):
Number of moles of
 =
=
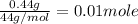
Since, in 1 mole of
 there is 1 mole of
there is 1 mole of
 .
.
So, number of moles of
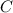 in 0.01 mole of
in 0.01 mole of
 is 0.01 mole.
is 0.01 mole.
Hence, the number of moles of
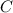 in 440 mg of
in 440 mg of
 is 0.01 mole.
is 0.01 mole.