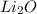The ionic character of any compound depend on the lattice energy as well as the electronegativity of element present in that compound.
More would be the lattice energy more would be ionic nature of that compound.
The lattice energy of any compound is inversely proportional to the ionic radii cation and anion.
In given case the ionic radii of oxide in both oxides would be equal therefore the lattice energy only depend on the ionic radii of cation.
As the radii of Magnesium less then radii of lithium therefore lattice energy of Magnesium oxide would be more than lithium oxide.
Hence, MgO would be more ionic in nature than