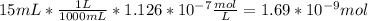pH of the solution before incubation = 7.65
![[H_(3)O^(+)]=10^(-7.65)=2.24*10^(-8) M](https://img.qammunity.org/2019/formulas/chemistry/college/z8ykcqq6le8dsgbkcfmb7y7xxjpwcypcfm.png)
pH after the solution was incubated with acetylcholinesterase = 6.87
After incubation,
![[H_(3)O^(+)]=10^(-6.87)=1.35*10^(-7) M](https://img.qammunity.org/2019/formulas/chemistry/college/23x0s8r1s7yb7gamnl1i2dvt3nm1w5yovk.png)
Difference in the concentration of hydronium ion =
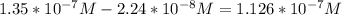
When solution containing acetylcholine is incubated with acetylcholinesterase, acetylcholine is converted to acetic acid and choline. So the decrease in the concentration of acetylcholine will be equal to the increase in the concentration of hydronium ion produced from acetic acid.
Moles of acetylcholine =