Mass number is equal to the sum of number of neutrons (neutral subatomic particle) and number of protons (positively charge particle).
Mass number =
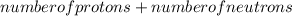
Now, atomic number is same as the number of protons i.e. atomic of nitrogen is 7 . Thus, number of protons is also equal to 7.
Therefore,
Mass number =
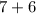
=
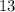
Hence, mass number of nitrogen is equal to
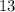 .
.