Answer:-
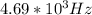
Solution:- Wavelength is given and we are asked to calculate the frequency. The equation that correlates frequency with wavelength is:
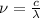
where,
 is the symbol for frequency, c is the symbol for speed of light and
is the symbol for frequency, c is the symbol for speed of light and
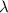 is the symbol for wavelength.
is the symbol for wavelength.
Speed of light is
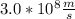 and the wavelength is given as
and the wavelength is given as
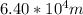 .
.
Let's plug in the values in the equation and so the calculations to calculate the frequency:
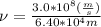
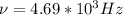
(since,
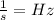 )
)
So, the frequency of the wave is
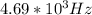 .
.